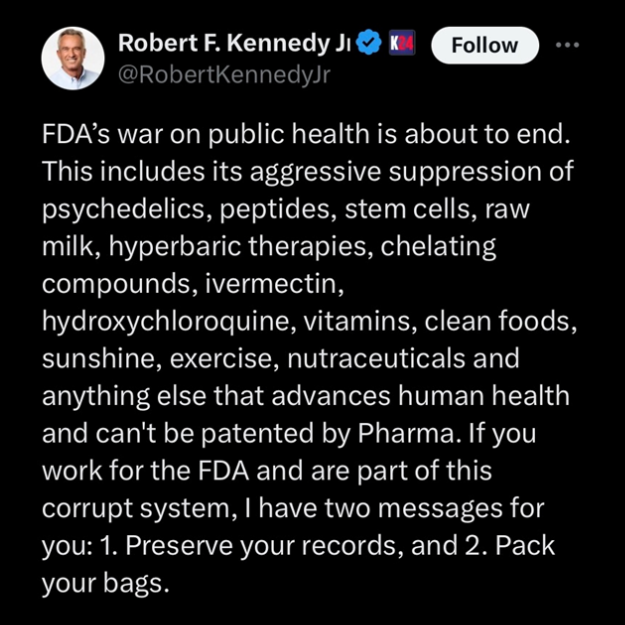
Robert F. Kennedy Jr. recently wrote, “FDA’s war on public health is about to end. This includes its aggressive suppression of…stem cell(s)…” He lists a number of other treatments and alternative medicines in his statement, but we will narrow the focus to our expertise which is stem cell research, cultivation, and clinical treatment.
With the USA Presidential Election decided, and the likelihood of an RFK Jr. appointment a reality, this note I referenced above has been shared with our team quite a bit and we are asked for comment on this hourly.
First, Auragens has from inception been an advocate for global acceptance of hu-MSC usage as a standard of care globally. Our protocols are all created to present clear and unbiased evidence of our safety, consistency, and successful use in a cross section of diagnosis. As one of the only fully integrated regenerative medical facilities who maintain laboratory, cell banks, clinicians, treatment rooms, and conduct research for publishing, under one roof we have a unique and one of a kind panorama of the need behind this deregulation. And the spirit of the statement is one we identify with.
We also, when asked, urge caution as stem cell treatments are a medical specialty. For safety there needs to remain extremely high standards and oversight. While becoming available to all in the USA and beyond is a good for all, it must be accomplished by preserving the safety profile and administration must be done by those specifically trained and experienced in doing so. For our patients who visited a US based “stem cell clinic,” prior to choosing Auragens, this will resonate as promises were made that were false. RFK Jr’s statement helps expose that the domestic market in the USA is not what it has purported itself to be and in fact the international market of which Auragens leads is the direction we must head. But naturally, internationally, not all facilities are created equal. Again, this is where Auragens is stepping in to establish the bar that all must clear.
We are cautiously optimistic that a door may be opened for greater availability of our product and practices. We are taking all steps to be able to make our cell bank and expertise available should this opportunity be opened in the USA. And we will continue to uphold the highest standards in the industry to ensure that all who are involved, or who wish to participate, provide a safe and effective product that is in the patient’s best interest.
And we look forward to continuing to work with the US based institutions, and their leadership, and Mr. Kennedy should that come to pass, to establish a safe, effective, and available treatment for all it may benefit.