Dr. James Utley PhD, Chief Scientific Officer at Auragens


OPTIMIZING CELLULAR THERAPY
Enhancing Patient Care with Advanced Cell Evaluation Process
At Auragens, our scientific team implements our proprietary process of immunophenotyping cells during expansion.
Prior to cells being administered to our patients, we evaluate: cell morphology, optimized cell surface marker selection, cell survivability and potency, and cell multiplication potential.
The result of this process is a safe, customized and high-performance cellular product.
We provide our patients an advanced alternative treatment option for accelerated healing and rapid recovery.
– Dr. James Utley PhD, Chief Scientific Officer
ELEVATING HEALTHCARE STANDARDS
Auragens Commitment to Stem Cell Excellence
Auragens is a leader in the stem cell field because we put our science and medical first. Creating the best cellular product in-house and using the best methods for its administration leads to the best results for our patients.
Dr. Utley, here, provides a brief overview of the cells we use and the reason for it. Auragens believes in transparency and sharing knowledge to benefit the field as a whole. The more we are able to breakthrough the misinformation the more effective we will be at making this treatment a standard of care for all who need it.
Dr. James Utley PhD, Chief Scientific Officer at Auragens

Umbilical mesenchymal stem cells (hUC-MSCs) are a type of stem cell that can be isolated from the umbilical cord tissue. The quality of hUC-MSCs can be assessed using a variety of criteria. At Auragens we follow ISCT guidelines and only use the highest quality hUC-MSCs, that we thoroughly evaluate to make sure it is pure, viable, multipotent, immunomodulatory, and capable of expansion.
The use of stem cells, including umbilical stem cells, is a complex medical procedure that requires specialized knowledge and training. The recommended dose and treatment protocol for umbilical stem cells can vary depending on factors such as the patient’s age, medical condition, and the specific goals of the treatment. At Auragens we achieve optimum volume products through understanding the complexity of the cellular expansion potential. Cellular expansion potential is important in that hUC-MSCs should be able to expand in culture while maintaining their stem cell properties. This factor makes Auragens exceptional in that this technique improves the ability to identify specific dosing criteria for the individual patient and the specific ailment.
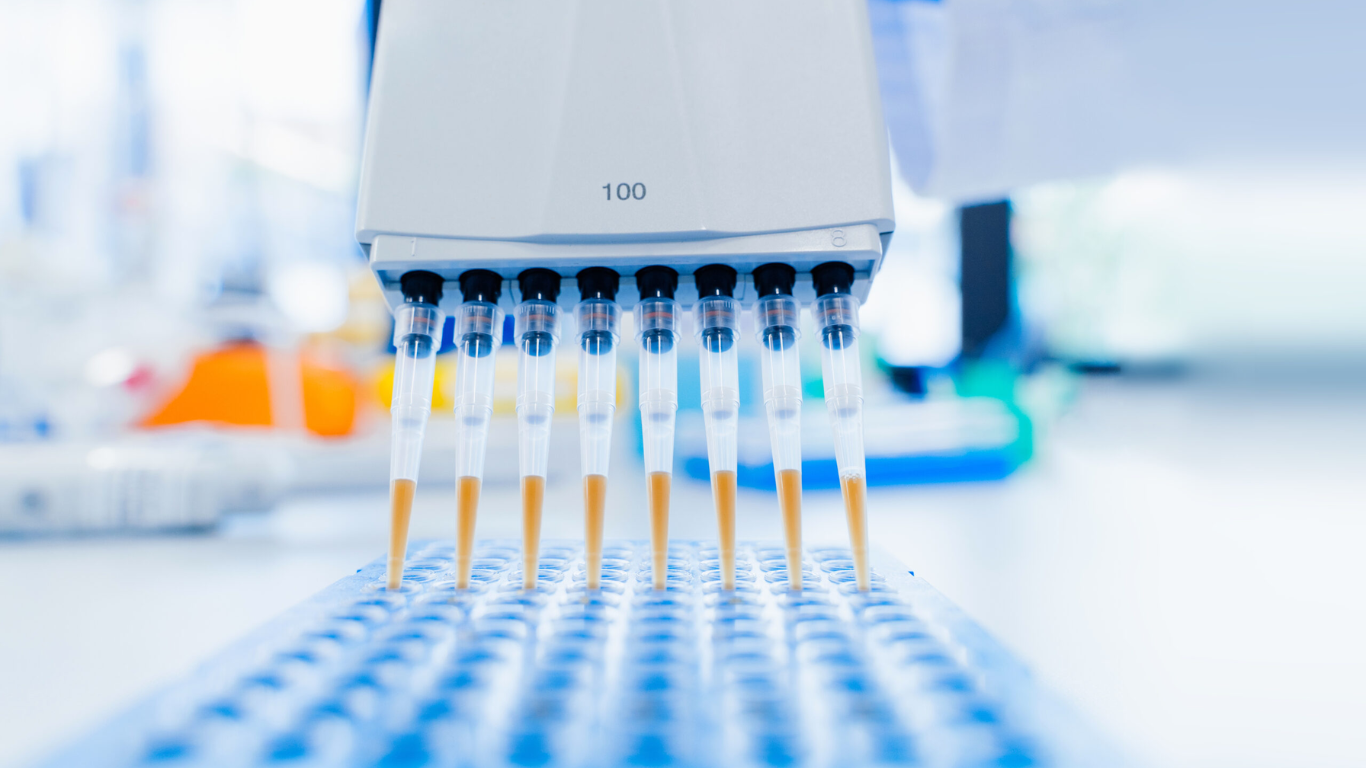

Auragens uses personalized medicine as we target specific populations of hUC-MSCs and ensure that they are as pure as possible, meaning they are free from contamination by other cell types. We use state of the art techniques such as 2D and 3D culturing, and flow cytometry to assess the purity of the cells we use for administration. Our Type selection process ensures that the hUC-MSCs are able to survive and thrive after being isolated from the umbilical cord tissue. A high percentage of viable cells is one of our key selection indicators of an effective product. In our Type selection process, we also ensure both Multipotency capability and Immunomodulatory properties are intact. The secret behind Auragens success is how we custom select the Stem Cells based on the Immunomodulatory properties. We know that hUC-MSCs should have the ability to modulate the immune system, so we use those properties and reinforce them with the latest technologies which can make them useful for treating certain diseases. We harness the natural capability of the cell to regenerate and renew to maximize its impact in the body.
We are not made of drugs, we are made of cells.”
– Cade Hildreth

